Headspace GC-MS
This technique can be excellent for detecting the most volatile compounds present in the fuel sample from propane/isobutanes upwards, and including contaminants such as chlorinated solvents, or other solvents such as ethanol, acetone, methyl acetate. What is detected can depend on sampling temperature, and instrumental conditions (especially GC) but it won’t show the whole profile of the fuel itself. That’s because it’s the vapour above the sample that is analysed, leaving the majority of the fuel sample sitting in the vial.
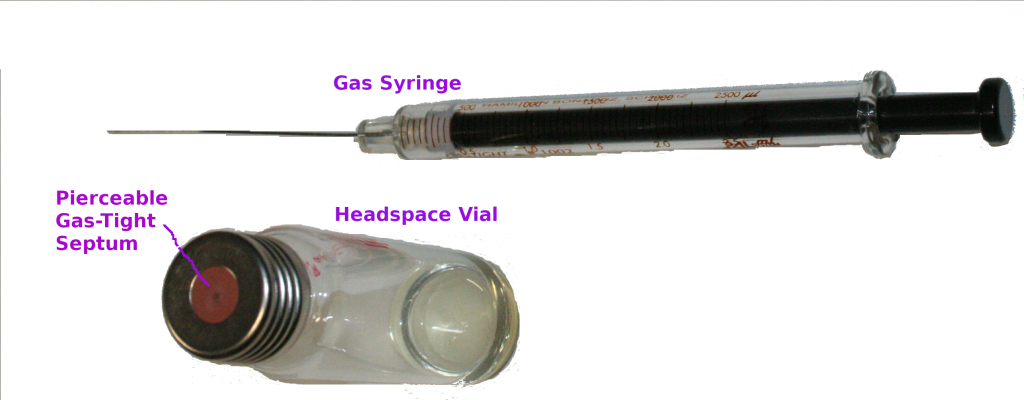
It can be useful for investigating low flashpoint problems, because the most volatile compounds are analysed:
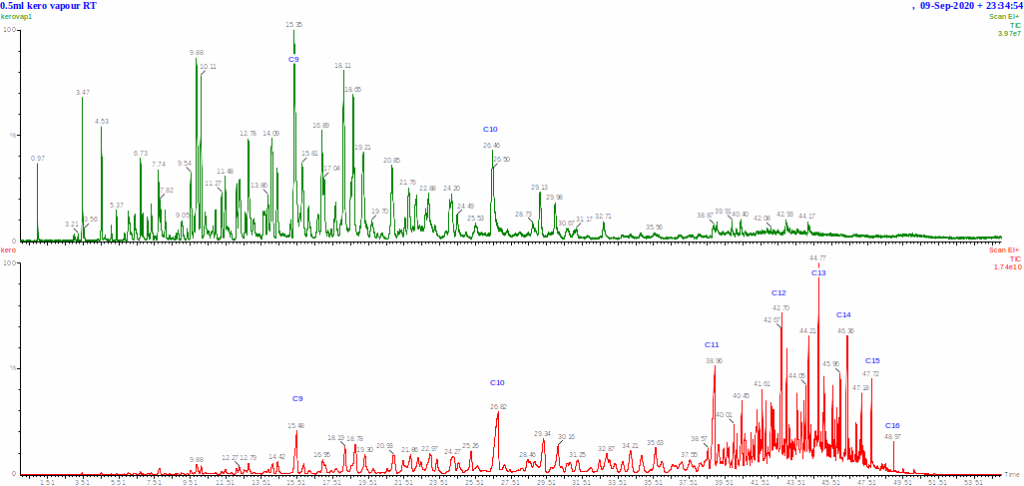
Chromatograms of a kerosene and its headspace at ambient temperature (both run under the same conditions) are shown above. You can see that the headspace contains the most volatile hydrocarbons but not the majority of the composition of the fuel. Note that the volume of liquid kerosene injected was about 1000 times less than the volume of vapour injected, and the sensitivity of the vapour chromatogram is about 500 times lower than the liquid kerosene injection.
Residual fuels have much lower concentrations of light hydrocarbons and hence produce much lower concentrations of vapour at room temperature or even 80-100°C. This means the hydrocarbon background will be lower allowing much easier detection of volatile contaminants. The chromatogram shown below was of the residual fuel’s headspace sampled at 70°C. This shows not only the most volatile components, but trace quantities of higher boiling components which start to exhibit significant vapour pressure at the sampling temperature.
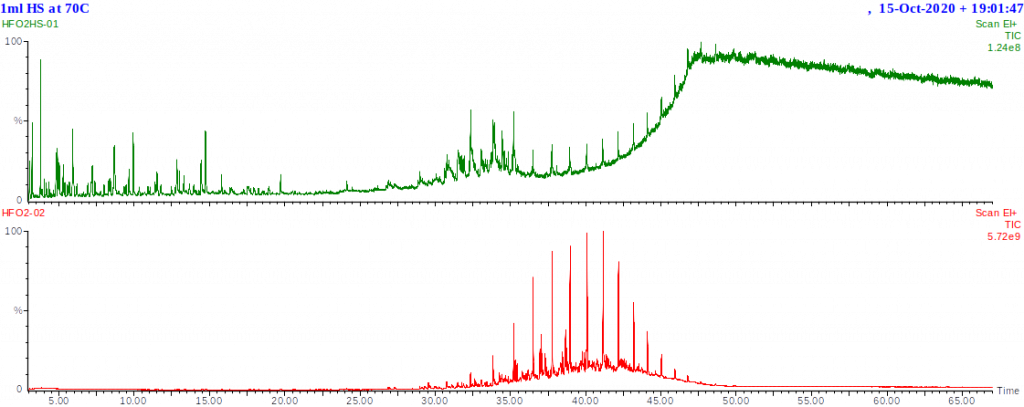
The hydrocarbon profile of residual fuel A’s headspace is actually quite similar to that of the kerosene shown above. The proportion of the fuel that is in the vapour state (i.e. headspace) is, however, much smaller for the residual fuel than for the kerosene at the same temperature.
We can inject much larger volumes of the headspace vapour compared to the direct injection of the fuel sample, and because there is not a large solvent peak volatile solvents such as chlorinated solvents (e.g. trichloroethylene), or volatile alcohols and esters can be seen.
