In this article we show how we examine Kerosene and Diesel fuel using GCMS.
Kerosene
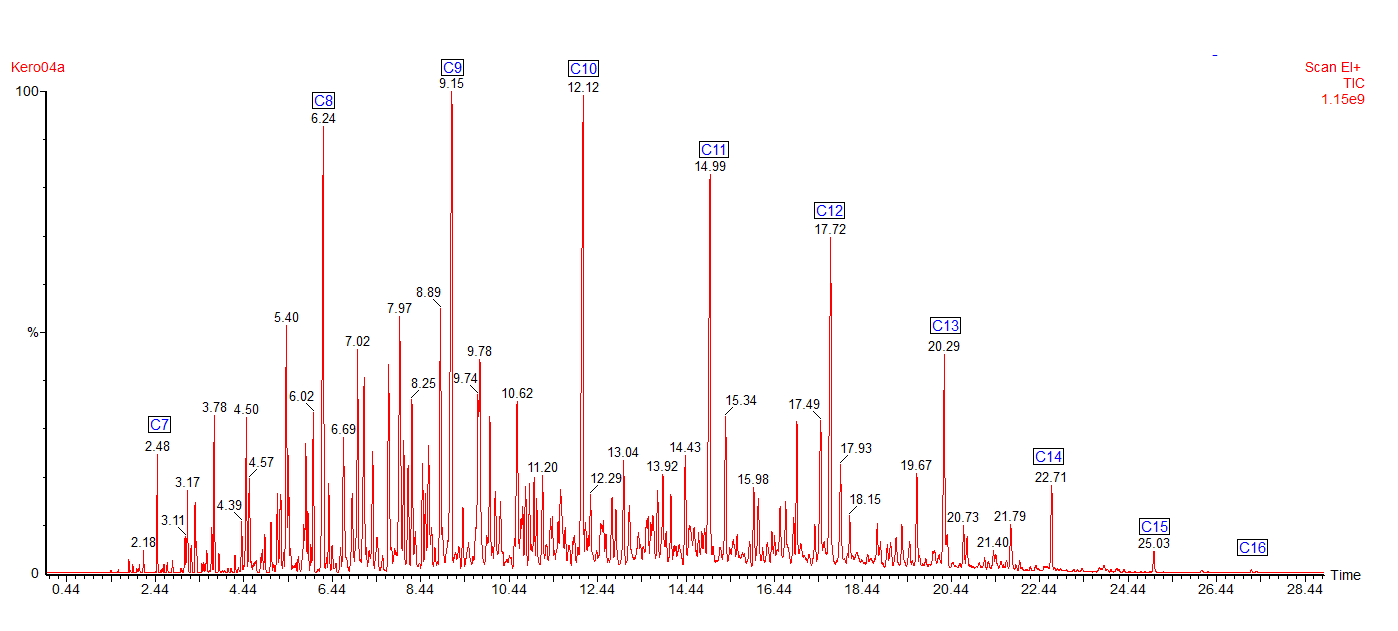
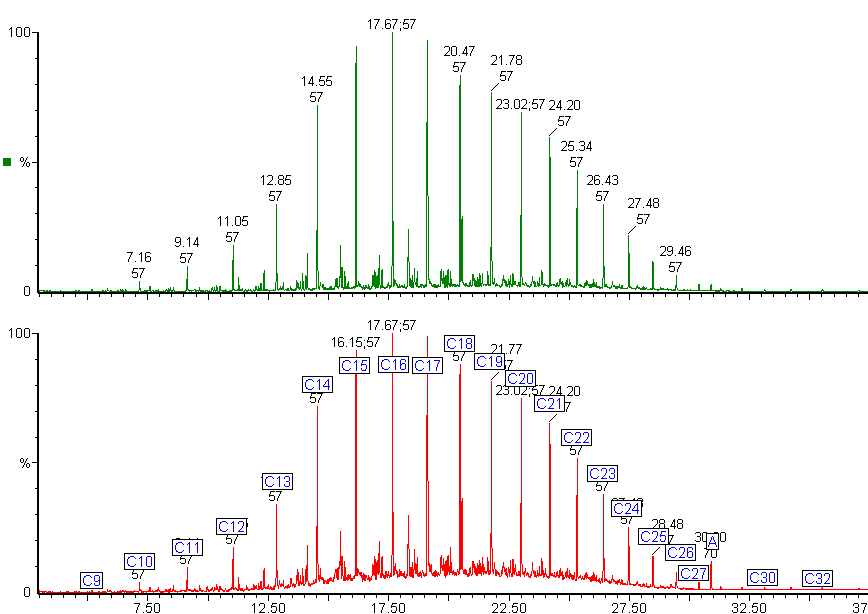
Here is a typical chromatogram of a kerosene using a Mass Spectrometer as the detector. The number and distribution of the peaks forms a fingerprint of the fuel.
The chromatogram shows all the major peaks showing that this kerosene has an alkane range from heptane (C7) to hexadecane (C16). Kerosene contains different types of hydrocarbons: alkanes (or paraffins), cycloalkanes (also called naphthenes) and aromatics.
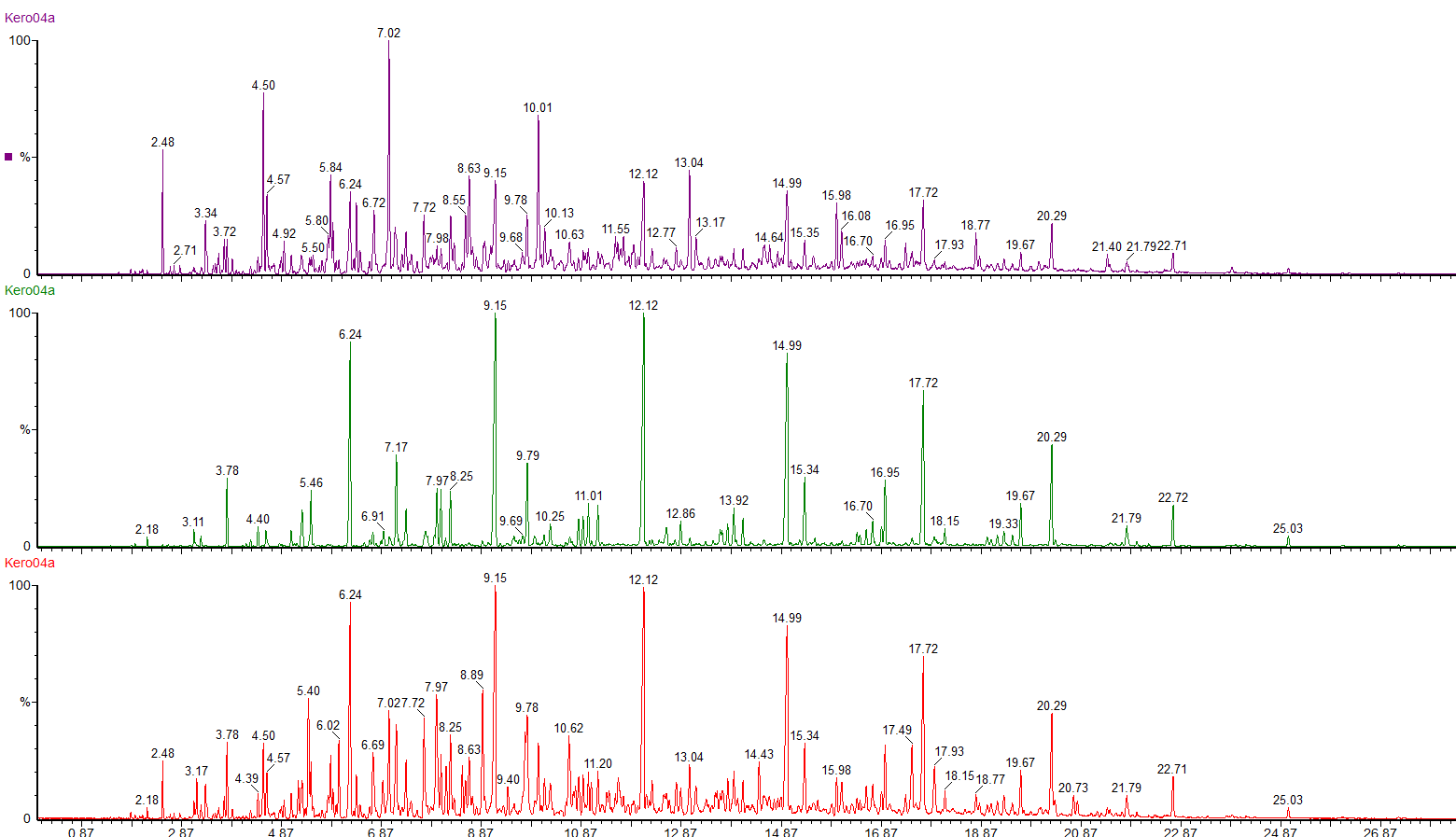
We can use selective ion monitoring to separate out these different classes of compounds.
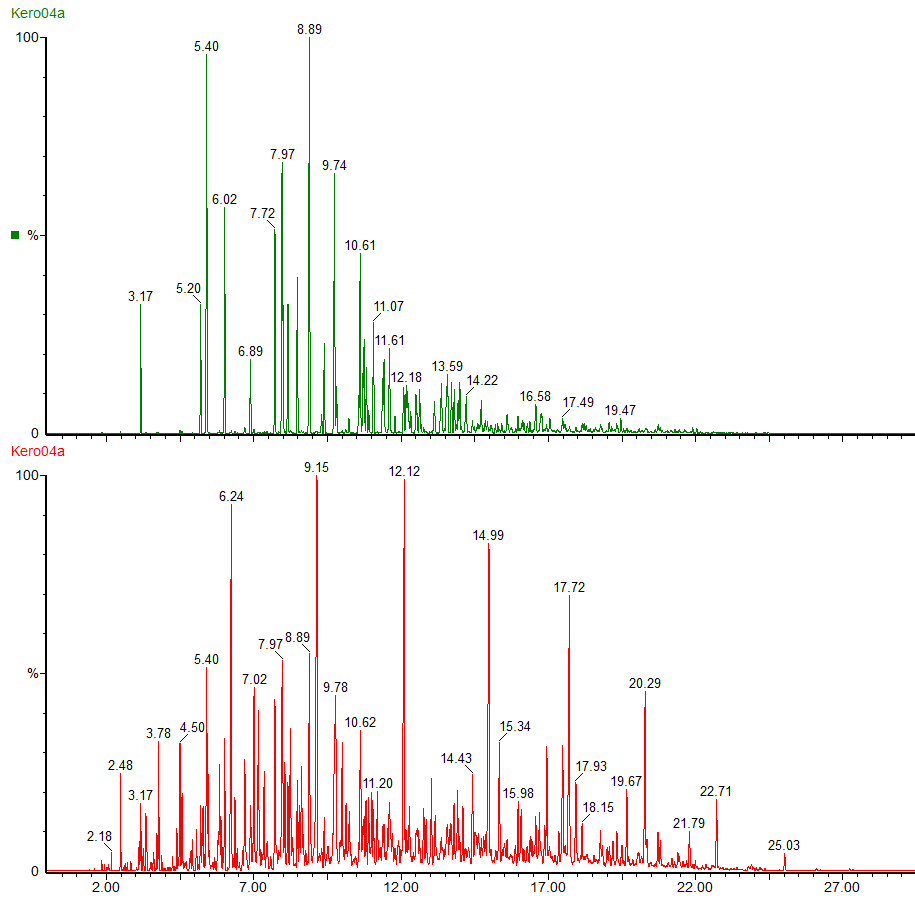
The red chromatogram shows the whole range of compounds in the kerosene. By using selected ions we can see the straight chain and branched chain alkanes (green chromatogram) and the profile of the cycloalkanes (purple chromatogram).
The green chromatogram shows the alkylbenzenes present in the kerosene, ranging from toluene to C6 alkyl benzenes.
Diesel Fuel
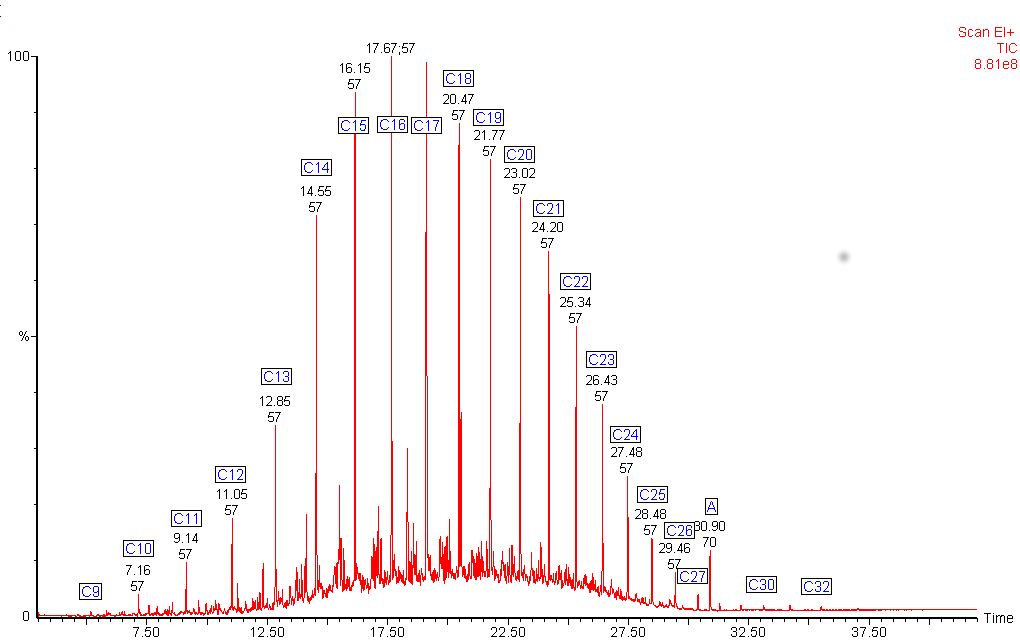
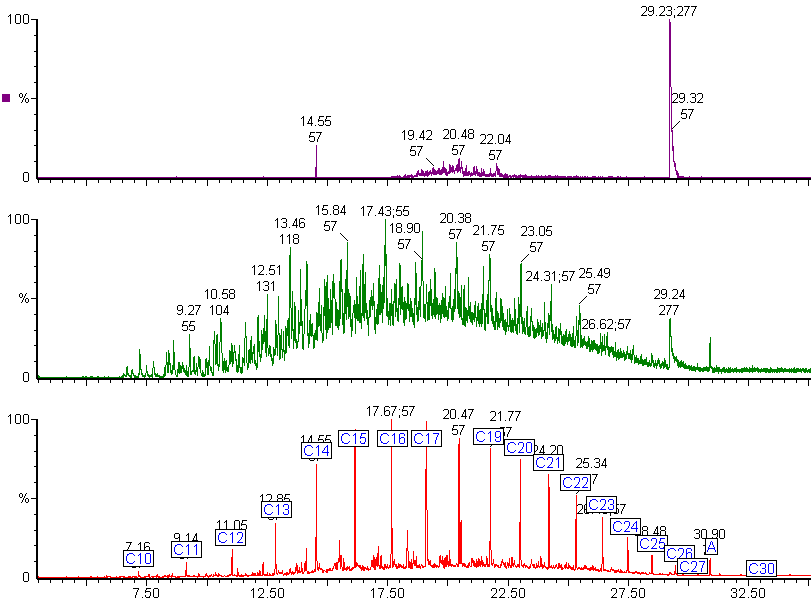
Diesel fuel shows a mixture of hydrocarbons with a wider boiling range.
The alkanes in this diesel fuel range from nonane (C9) to dotriacontane (C32). The chromatogram shows an unexpected peak (labelled A). The mass spectrum from this peak identified it as di-octyl phthalate, a common plasticiser often found in plastic containers.
The green chromatogram shows the alkane distribution in the diesel fuel.
Sometimes contaminants are not seen in the main chromatogram because the hydrocarbons overlap the contaminant peak.
Selective ion monitoring can reveal contaminants.
The green chromatogram shows the selected ion chromatogram for alkyl benzenes. The peak at 29.24 min is unusual and indicates a possible contaminant. By checking the mass spectrum of this peak against the NIST mass spectral library, we found a match with triphenylphosphine oxide, which is not a normal component of diesel fuel.
The purple chromatogram shows the same peak is present in the chromatogram produced for the two main fragment ions for triphenylphosphine oxide.